Embark on a journey into the fascinating realm of mixture composition, where we delve into the intricacies of calculating mass and mole fractions, exploring real-world applications, and visualizing data through compelling graphs and tables. Composition of Mixtures Practice Problems serves as your ultimate companion, providing a comprehensive guide to understanding and mastering this fundamental concept.
Throughout this exploration, we will encounter a diverse range of practice problems that challenge our understanding of mixture composition. From determining mass fractions to calculating mole fractions, each problem is meticulously crafted to enhance our problem-solving skills and deepen our comprehension of the subject matter.
1. Understanding Mixture Composition
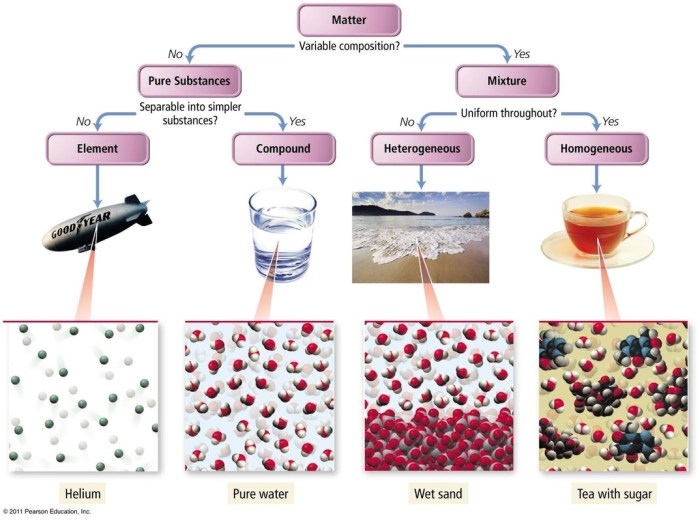
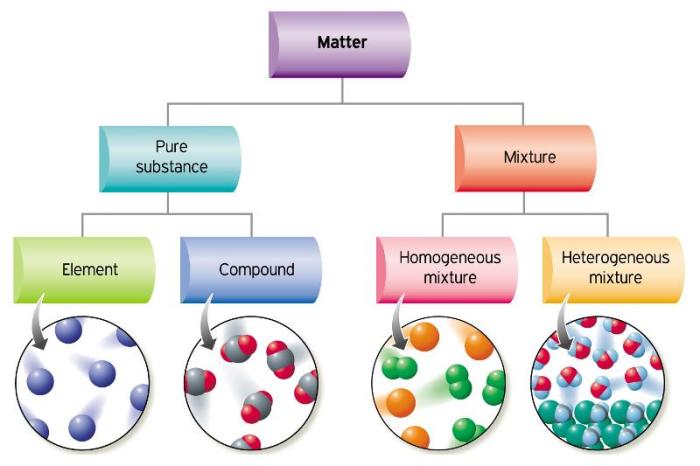
A mixture is a combination of two or more chemical substances that are not chemically bonded. The components of a mixture retain their identity and are mixed in different forms, such as solutions, suspensions, or colloids.
The composition of a mixture can be expressed in terms of mass fraction or mole fraction. Mass fraction is the ratio of the mass of a component to the total mass of the mixture, while mole fraction is the ratio of the number of moles of a component to the total number of moles in the mixture.
The relationship between mass, moles, and composition can be expressed using the following formulas:
Mass fraction = mass of component / total mass of mixtureMole fraction = moles of component / total moles of mixture
2. Calculating Mixture Composition: Composition Of Mixtures Practice Problems
Mass Fraction, Composition of mixtures practice problems
- To calculate mass fraction from mass and moles, use the formula: Mass fraction = mass of component / total mass of mixture
- To calculate mass fraction from moles and mass, use the formula: Mass fraction = (moles of component x molar mass of component) / total mass of mixture
Mole Fraction
- To calculate mole fraction from moles and mass, use the formula: Mole fraction = moles of component / total moles of mixture
- To calculate mole fraction from mass and moles, use the formula: Mole fraction = (mass of component / molar mass of component) / total moles of mixture
Converting between Mass and Mole Fractions
- To convert from mass fraction to mole fraction, use the formula: Mole fraction = mass fraction / (mass fraction x molar mass of component)
- To convert from mole fraction to mass fraction, use the formula: Mass fraction = mole fraction x molar mass of component
General Inquiries
What is the difference between mass fraction and mole fraction?
Mass fraction represents the ratio of the mass of a component to the total mass of the mixture, while mole fraction represents the ratio of the number of moles of a component to the total number of moles in the mixture.
How can I convert between mass fraction and mole fraction?
To convert from mass fraction to mole fraction, multiply the mass fraction by the molar mass of the component and divide by the total mass of the mixture. To convert from mole fraction to mass fraction, multiply the mole fraction by the molar mass of the component and divide by the total number of moles in the mixture.
What are some real-world applications of mixture composition?
Mixture composition plays a crucial role in fields such as chemistry, engineering, and medicine. In chemistry, it helps determine the properties of mixtures and predict their behavior. In engineering, it is used to design and optimize processes involving mixtures. In medicine, it aids in formulating drugs and understanding their interactions within the body.